Morinaga Milk Industry Co., Ltd. is a leading dairy product company in Japan with over a century of history harnessing the nutritional properties of dairy products and its functional ingredients. Morinaga Milk is also a key global probiotics manufacturer that offer a premium line of probiotics bifidobacteria of high compatibility with humans – Human-Residential Bifidobacteria (HRB). Morinaga Milk excels in innovative technology and offers various dairy probiotics and other beneficial functional ingredients to customers throughout the world.
Morinaga Milk holds a leading marketing position with its many top brands in yogurt, milk, infant formula, beverage, cheese, butter, ice cream, pudding, dietary supplements and clinical foods. The functional ingredients manufactured and supplied globally by Morinaga Milk include HRB probiotics, postbiotics LAC-Shield, lactoferrin, lactulose, whey proteins, and peptides, among which are used in high value-added nutritional products such as infant formulas, dietary supplements and functional foods.
Morinaga Milk started research of Human-Residential Bifidobacteria (HRB) in the 1960s, inspired by the fact that bifidobacteria are the predominant bacteria residing in the intestines of breast-fed infants. Below are the main strains of Morinaga Milk’s HRB probiotics.
BB536: well-documented multifunctional probiotics
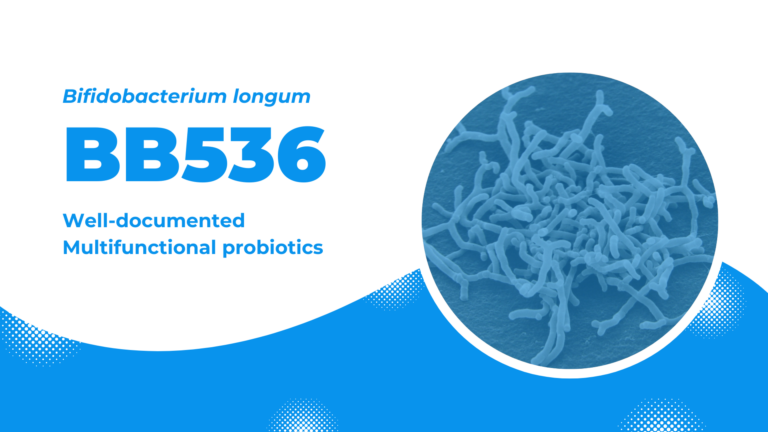
Bifidobacterium longum BB536, the flagship probiotics of Morinaga Milk, is one of the most well-established, clinically effective probiotic strain that confers numerous profound beneficial effects on humans. BB536 possesses a proven track record of safety and clinical efficacy in improvement of intestinal health, maintenance of intestinal microflora balance, regulation of immune response, anti-allergy, and protection against microbial infections, as demonstrated in more than 220 scientific studies (as of March 2022).
Regulatory status of BB536:
1996 Japan FOSHU status
2009 FDA notified GRAS status for foods
2019 FDA notified GRAS status for infants
2022 China New Food Ingredient for use in infant and toddler foods
M-16V: ideal probiotics for infant health
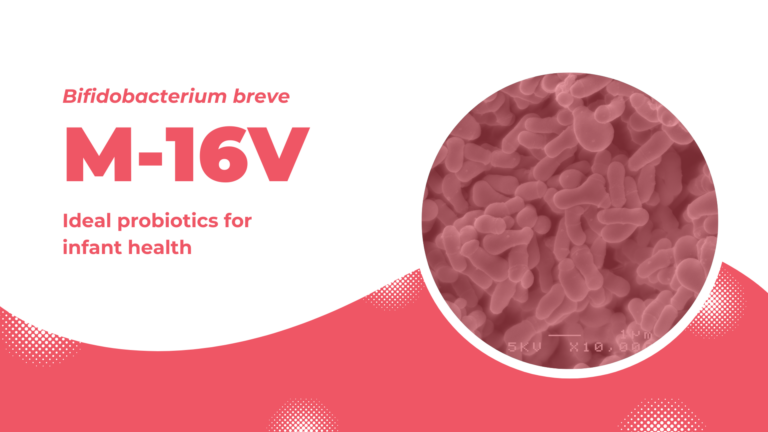
Bifidobacterium breve M-16V is one of the best studied clinically effective probiotic strains that exert positive effects, particularly in infants, to support healthy growth and promote well-being. Its safety and effect to promote healthy growth of premature infants are highly evaluated by medical professionals. To date, M-16V is used in more than 150 Neonatal Intensive Care Units (NICUs) in affiliated hospitals in Japan, Australia, New Zealand, and Singapore.
Regulatory status of M-16V:
2013 FDA notified GRAS status for foods
2013 FDA notified GRAS status for infants
2016 China authorized probiotic strains for infant’s food
M-63: probiotic for infant use and beneficial for healthy mood

Bifidobacterium infantis M-63 is unique among gut bacteria in its immense capacity to utilize human milk oligosaccharide (HMOs), the component that is highly abundant in human breast milk. HMOs offer no direct nutritive value for infants, but they function in shaping a better infant gut microbiota with life-long impacts. M-63 possesses superior potential to colonize and adapt to the intestinal environment of humans, especially infants. In addition, M-63 also shows potential in improving mental state in individuals with irritable bowel syndrome (IBS).
B-3: a unique probiotic for weight management
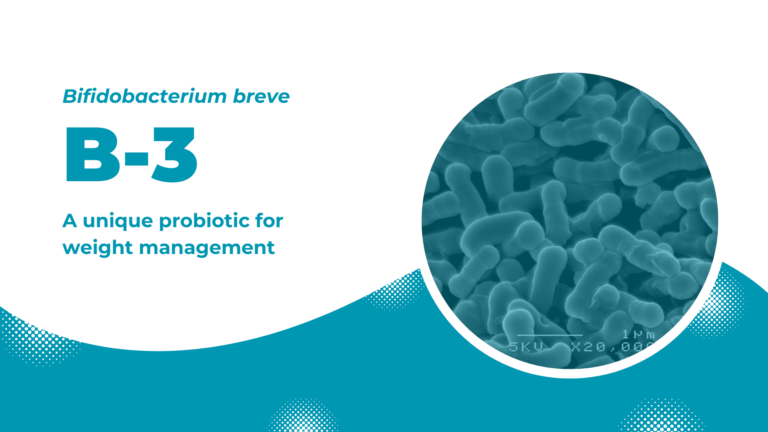
Bifidobacterium breve B-3 is a unique probiotic strain developed by focusing on the relationship between gut microbiota and metabolic syndrome. B-3 possesses an attractive effect in maintaining healthy body weight and ultimately improving one’s lifestyle.


